
Publications (Independent at SDU since 2012)
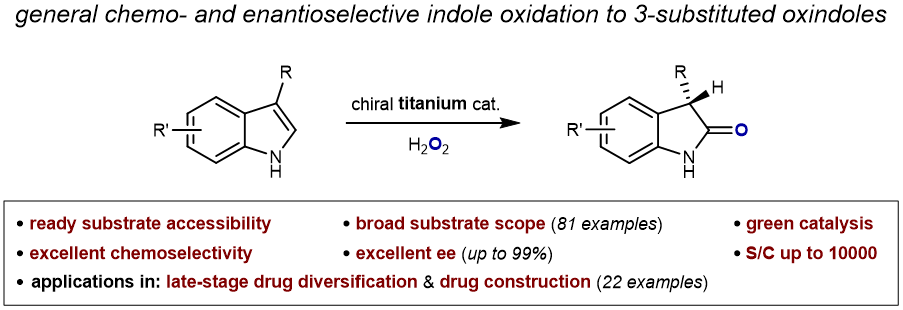
77. Late-Stage Chemo- and Enantioselective Oxidation of Indoles to C3-Monosubstituted Oxindoles. Li, S.; Liu, X.*; Tung, C.-H.; Liu, L.* J. Am. Chem. Soc. 2023, 145, 27120. link
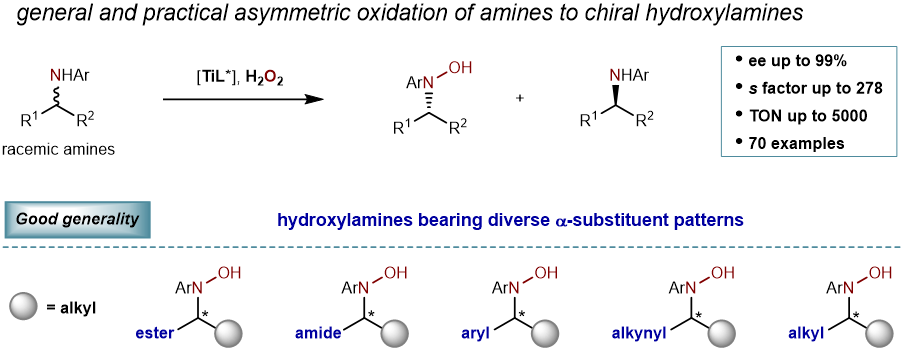
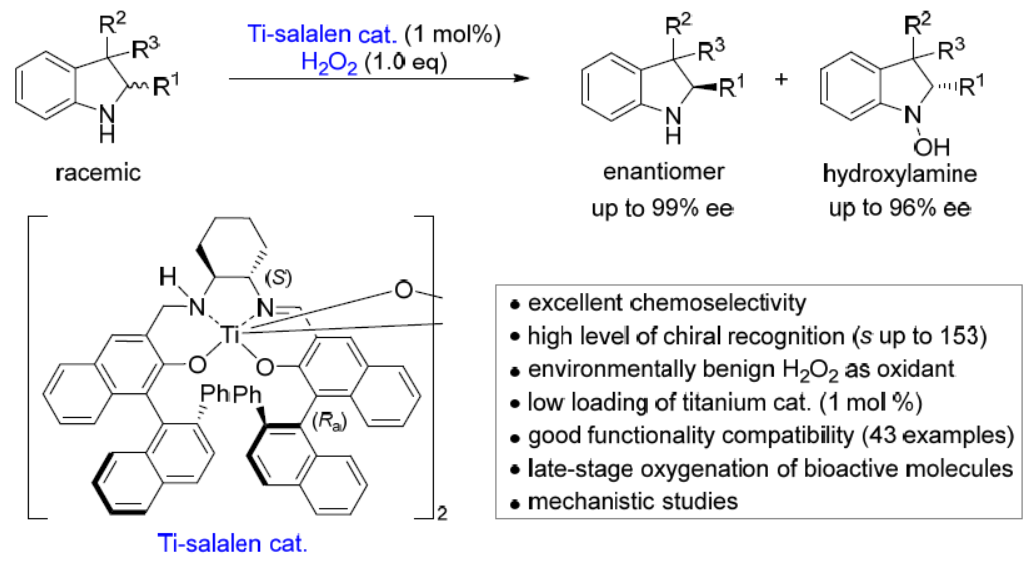
76. Catalytic Asymmetric Oxidation of Amines to Hydroxylamines. Wang, G.; Chen, T.; Jia, K.; Ma, W.; Tung, C.-H.; Liu, L.* J. Am. Chem. Soc. 2023, 145, 22276. link
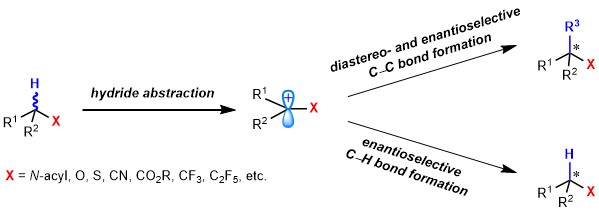
75. Hydride Abstraction Initiated Catalytic Stereoselective Intermolecular Bond-Forming Processes. Liu, L.* Acc. Chem. Res. 2022, 55, 3537. link
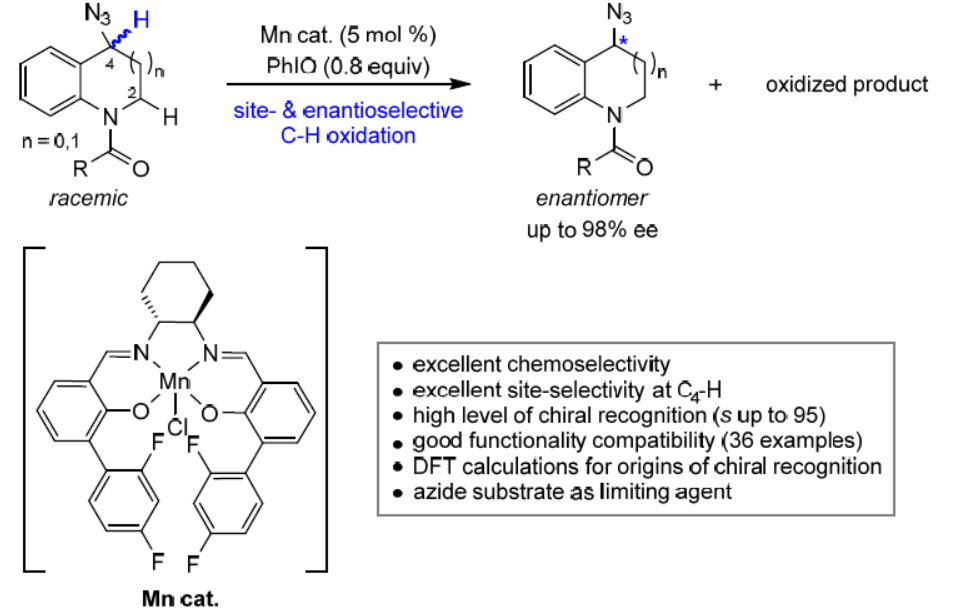
74. Site- and Enantioselective Manganese-Catalyzed Benzylic C-H Azidation of Indolines. Cao, M.; Wang, H.; Ma, Y.; Tung, C.-H.; Liu, L.* J. Am. Chem. Soc. 2022, 144, 15383. link

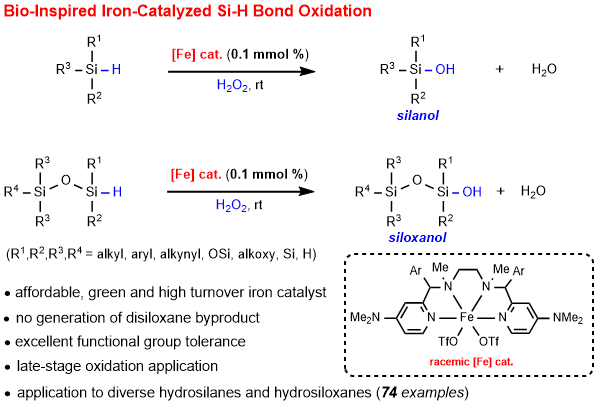
73. Practical and Selective Bio-Inspired Iron-Catalyzed Oxidation of Si-H Bonds to Diversely Functionalized Organosilanols. Li, S.; Li, H.; Tung, C.-H.; Liu, L.* ACS Catal. 2022, 12, 9143. link
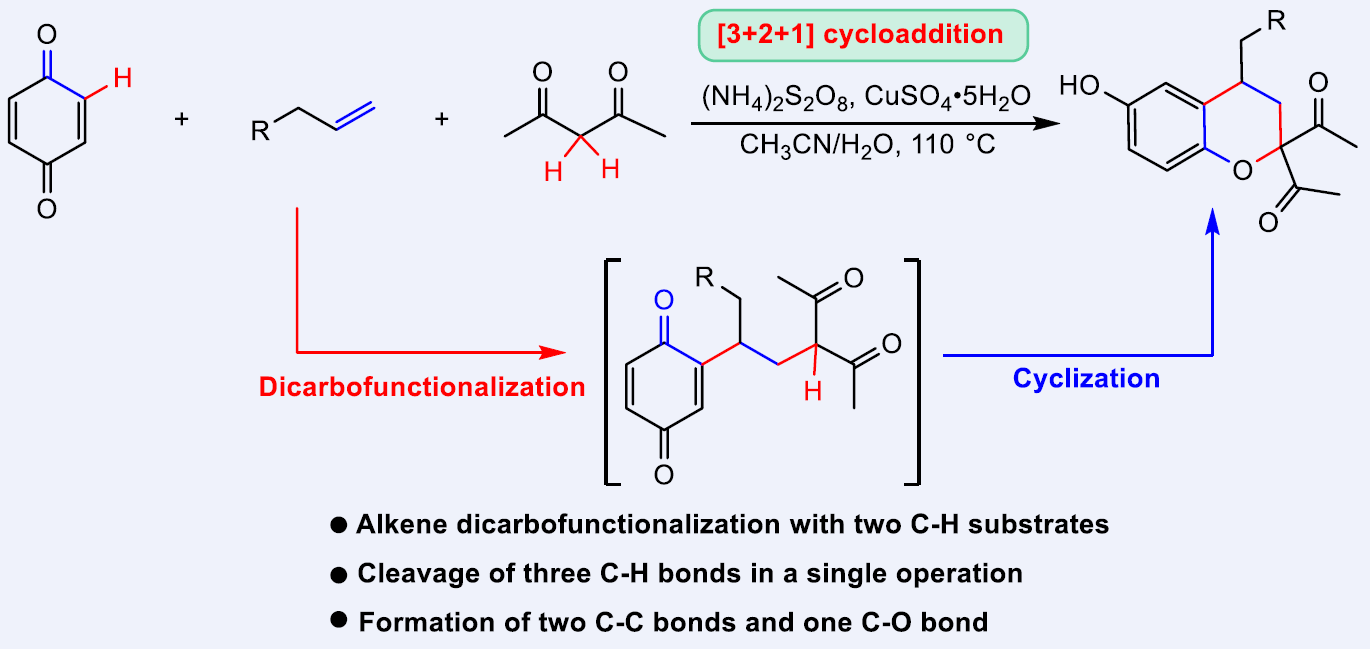
72. Copper-Catalyzed [3+2+1] Cycloaddition of Akenes with Benzoquinones and Dicarbonyl Compounds via Tandem Oxidative Dicarbofunctionalization/Cyclization Sequence. Du, T.; Li, S.; He, Y.; Long, H.; Liu, X.*; Li, H.-B.*; Liu, L.* Chin. J. Chem. 2022, 40, 1681. (invited contribution to the special issue of "Emerging Investigators in 2022") link
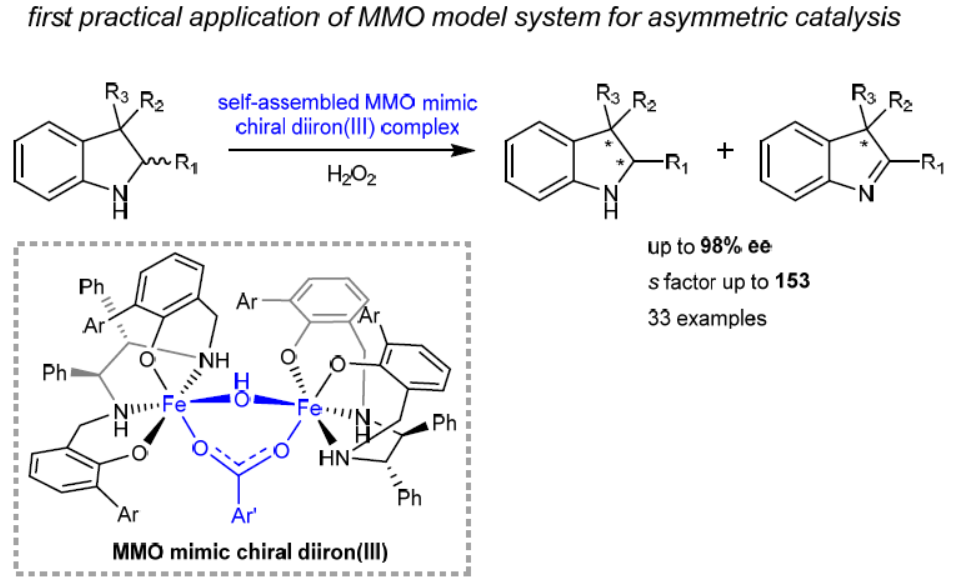
71. Methane Monooxygenase Mimic Asymmetric Oxidation: Self-Assembling μ-Hydroxo, Carboxylate-Bridged Diiron(III) Catalyzed Enantioselective Dehydrogenation. Guan, H.; Tung, C.-H.; Liu, L.* J. Am. Chem. Soc. 2022, 144, 5976. link
70. Kinetic resolution of cyclic benzylic azides enabled by site- and enantioselective C(sp3)-H oxidation. Ye, P.; Feng, A.; Wang, L.; Cao, M.; Zhu, R.; Liu, L.* Nat. Commun. 2022, 13, 1621. link Highlighted by Synform, 2022, A110. link
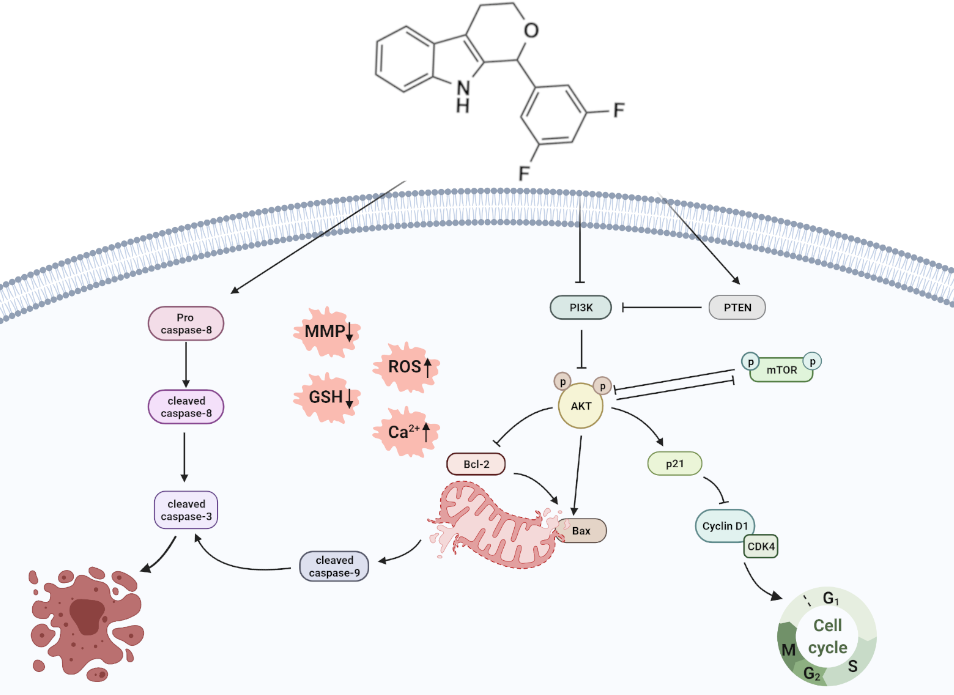
69. Design, synthesis and biological evaluation of novel 1,3,4,9-tetrahydropyrano[3,4-b]indoles as potential treatment of triple negative breast cancer by targeting PI3K/AKT/mTOR pathway. Qin, J.; Sun, X.; Ma, Y.; Cheng, Y.; Ma, Q.; Jing, W.; Qu, S.*; Liu, L.* Bioorg. Med. Chem. 2022, 55, 116594. link
68. Enantioselective Transfer Hydrogenation of Oxocarbenium Ions Enables Asymmetric Access to α-Substituted 1,3-Dihydroisobenzofurans. Zhou, L.; Jia, K.; Liu, X.*; Liu, L.* Synthesis 2022, 421. link
67. Enantioselective Construction of Single and Vicinal All-Carbon Quaternary Stereocenters through Ion Pair Catalyzed 1,6-Conjugate Addition. Zhu, Y.; Wang, H.; Wang, G.; Wang, Z.; Liu, Z.*; Liu, L.* Org. Lett. 2021, 23, 7248. link
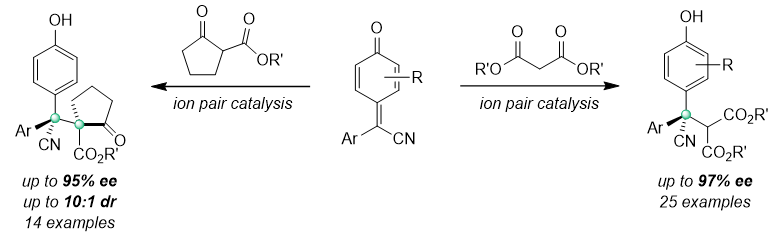
66. Construction of Vicinal Quaternary Carbon Stereocenters Through Diastereo- and Enantioselective Oxidative 1,6-Conjugate Addition. Liu, X.; Zhao, C.; Zhu, R.; Liu, L.* Angew. Chem. Int. Ed. 2021, 60, 18499. link
65. NaOtBu-catalyzed Hydrophosphonylation of δ-CN-δ-Aryl-Disubstituted Para-Quinone Methides with Phosphine Oxides. Wang, D.; Kan, L.; Ma, Y.*; Liu, L.* Chin. J. Org. Chem. 2021, 41, 3192. link
64. Kinetic resolution of indolines by asymmetric hydroxylamine formation. Wang, G.; Lu, R.; He, C.; Liu, L.* Nat. Commun. 2021, 12, 2512. link
63. Synthesis of diarylmethanes bearing CF3- and CN-substituted all-carbon quaternary centers and diarylmalononitriles via cyanation of δ-disubstituted Para-quinone methides. Pan, X.; Cao, M.; Li, S.; Wang, H.*; Liu, X.*; Liu, L.* Eur. J. Org. Chem. 2021, 1643. link
62. Redox deracemization of α-substituted 1,3-dihydroisobenzofurans. Chen, X.; Zhao, R.; Liu, Z.; Sun, S.; Ma, Y.; Liu, Q.*; Sun, X.*; Liu, L.* Chin. Chem. Lett. 2021, 32, 2305. link

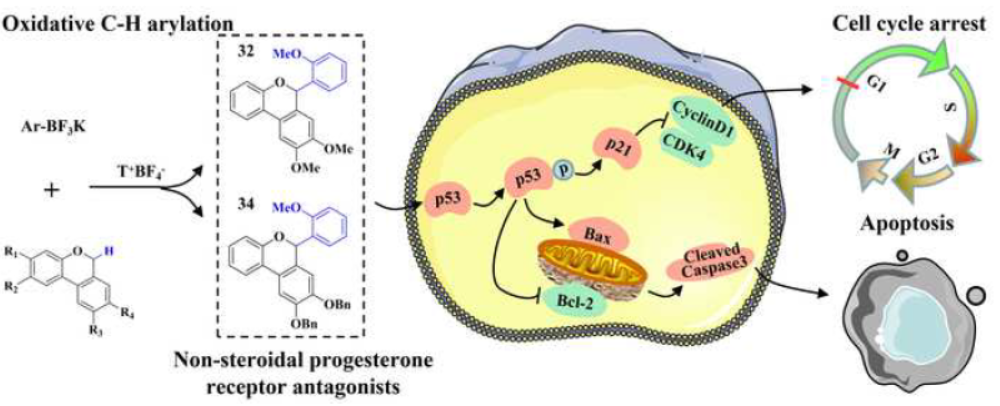
61. Rational design and synthesis of 6-aryl-6H-benzo[c] chromenes as non-steroidal progesterone receptor antagonists for the use against cancers. Qin, J.; Qu, S.; Cheng, Y.; Pan, G.; Jing, W.; Liu, X.; Sun, X.*; Liu, L.* Bioorg. Med. Chem. 2021, 32, 116003. link
60. Site- and Enantio-differentiating C(sp3)–H Oxidation Enables Asymmetric Access to Structurally and Stereochemically Diverse Saturated Cyclic Ethers. Sun, S.; Yang, Y.; Zhao, R.; Zhang, D.*; Liu, L.* J. Am. Chem. Soc. 2020, 142, 19346. link
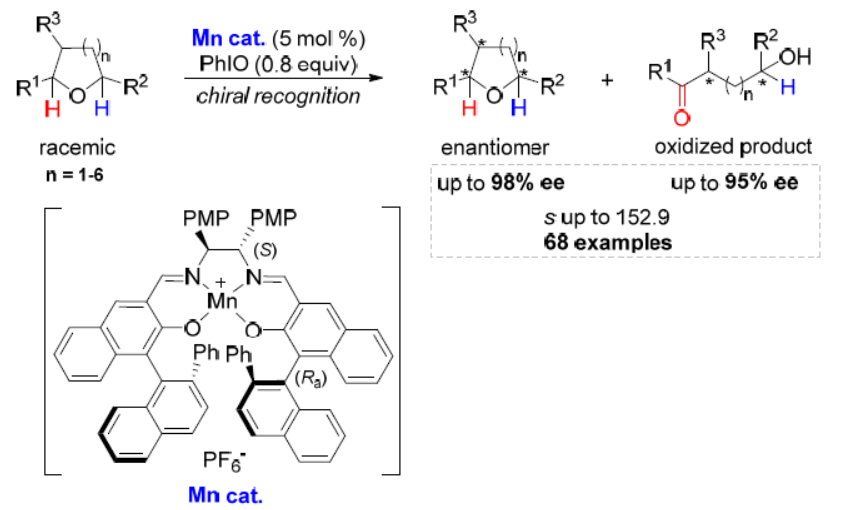
59. Oxidative Kinetic Resolution of Cyclic Benzylic Ethers. Sun, S.; Ma, Y.; Liu, Z.; Liu, L.* Angew. Chem. Int. Ed. 2021, 60, 176. link
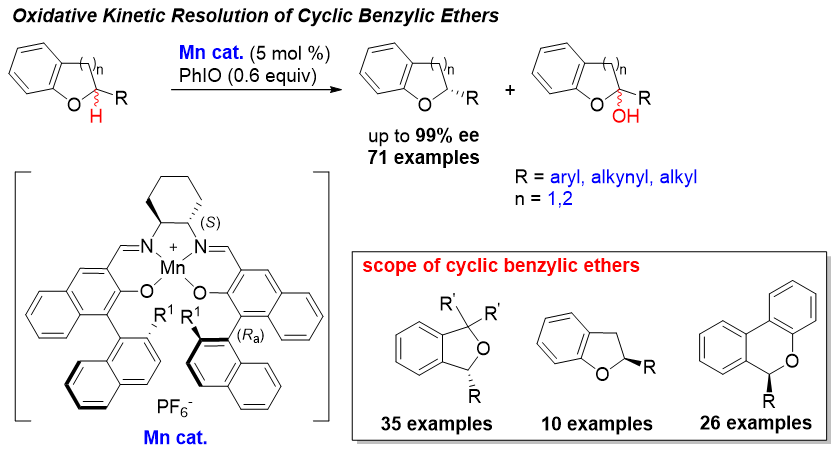
58. Nickel(II)-Catalyzed Asymmetric Alkylation of Acyclic Oxocarbenium Ions with Carboxylic Acid Derivatives. Ye, P.; Liu, X.; Wang, G.; Liu, L.* Chin. Chem. Lett. 2021, 32, 1237. link
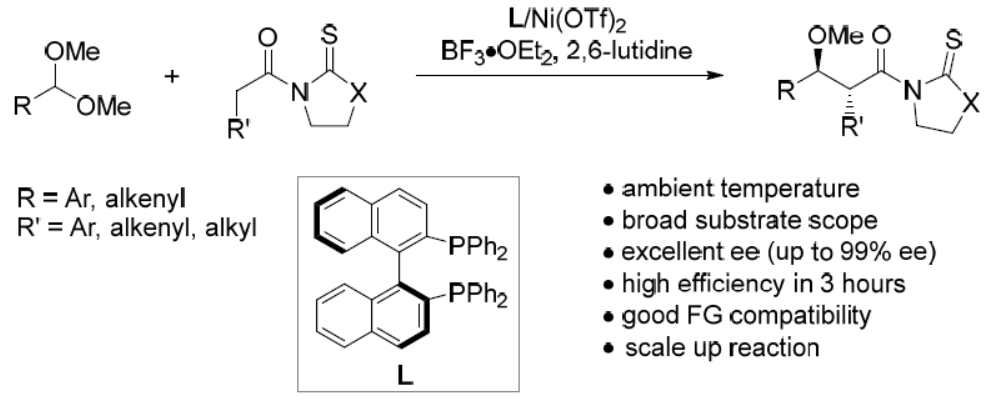
57. Redox Deracemization of Diarylmethyl Alkynes. Ma, Y.; Liu, X.; Mao, Y.; Huang, J.; Ma, S.*; Liu, L.* Org. Chem. Front. 2020, 7, 2526. link
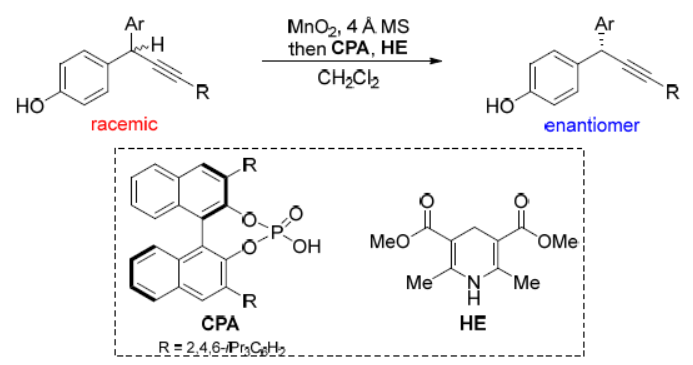
56. Synthesis of Unsymmetric Triarylmethanes Bearing CF3-Substituted All-Carbon Quaternary Stereocenters: 1,6-Arylation of delta-Trifluoromethyl Substituted para-Quinone Methides. Ma, Y.; Pang, J.; Pan, X.; Ma, S.*; Liu, X.*;Liu, L.* Synlett 2020, 31, 1619. link
55. Catalytic Asymmetric Alkylation of 2-Alkoxyl-tetrahydrofurans. Liu, X.; Sun, S.; Wang, G.; Bai, Z.; Pang, J.*; Liu, L.* Org. Chem. Front. 2020, 7, 2202. link
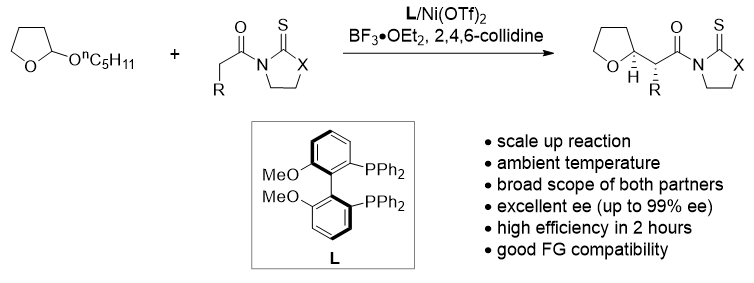
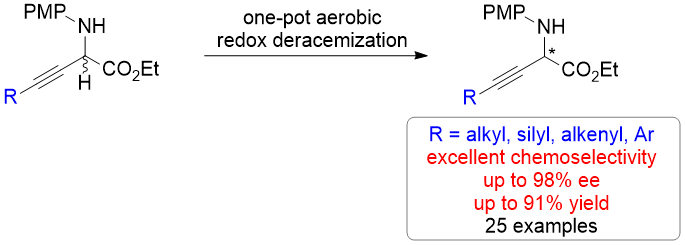
54. Redox Deracemization of Tertiary Stereocenters Adjacent to An Electron-withdrawing Group. Mao, Y.; Wang, Z.; Wang, G.; Zhao, R.; Kan, L.; Pan, X.; Liu, L.* ACS Catal. 2020, 10, 7785. link
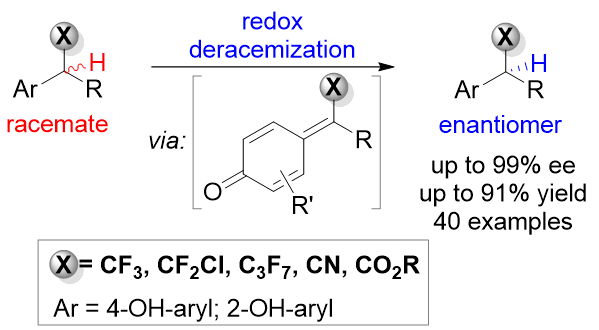
53. Catalytic Asymmetric Synthesis of β,γ-Alkynyl α-Amino Esters via Chemo- and Enantioselective Transfer Hydrogenation. Zhang, L.; Liu, A.; Liu, H.; Wan, R.*; Sun, S.*; Liu, L.* Chin. J. Org. Chem. 2020, 40, 2904. link
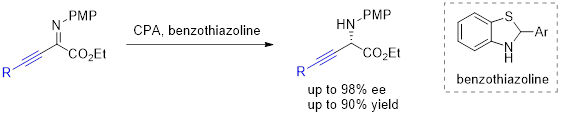
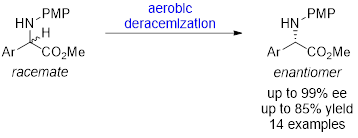
52. Aerobic Redox Deracemization of α-Aryl Glycine Esters. Chen, X.; Yan, L.; Zhang, L.; Zhao, C.; Feng, G.; Chen, L.; Sun, S.*; Liu, Q.*; Liu, L.* Tetrahedron Lett. 2020, 61, 152107. link
51. Synthesis of Sterically Hindered α-Aminonitriles through 1,6-Aza-Conjugate Addition of Anilines to δ-Cyano Substituted para-Quinone Methides. Wang, L.; Wang, N.; Qi, Y.; Sun, S.; Liu, X.*; Li, W.*; Liu, L.* Chin. J. Org. Chem. 2020, 40, 3934. link (“庆祝《有机化学》创刊四十周年专辑”约稿).
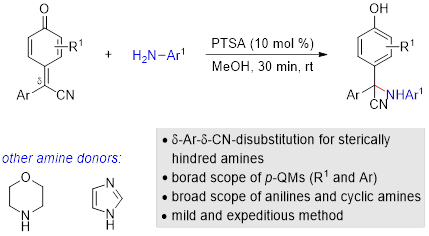
50. δ-Cyano substituted para-quinone methides enables access to unsymmetric tri- and tetraarylmethanes containing all-carbon quaternary stereocenters. Qi, Y.; Zhang, F.; Wang, L.; Feng, A.; Zhu, R.; Sun, S.*; Li, W.*; Liu, L.* Org. Biomol. Chem. 2020, 18, 3522. link
49. Redox deracemization of β,γ-alkynyl α-amino esters. Zhang, L.; Zhu, R.; Feng, A.; Zhao, C.; Chen, L.; Feng, G.; Liu, L.* Chem. Sci. 2020, 11, 4444. link
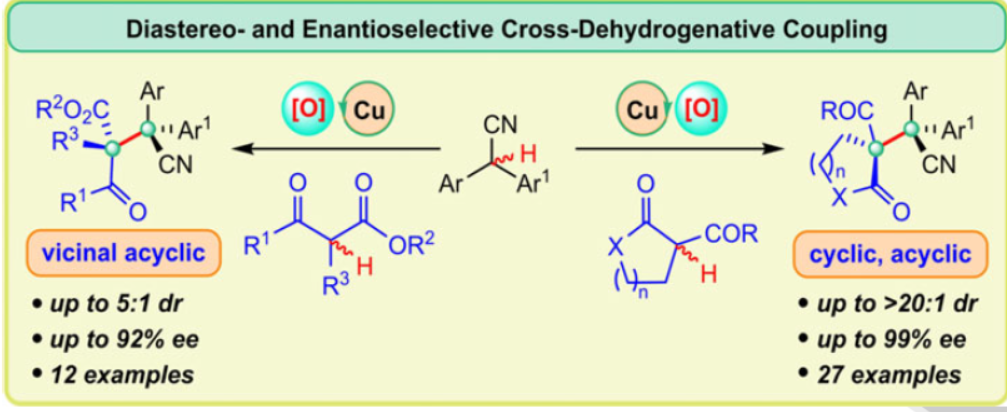
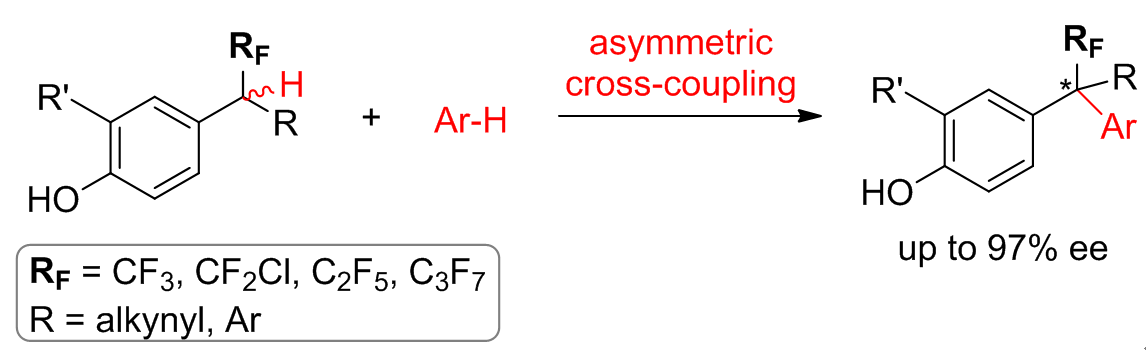
48. Cross-dehydrogenative coupling enables enantioselective access to CF3-substituted all-carbon quaternary stereocenters. Pan, X.; Wang, X.; Kan, L.; Mao, Y.; Zhu, Y.; Liu, L.* Chem. Sci. 2020, 11, 2414. link
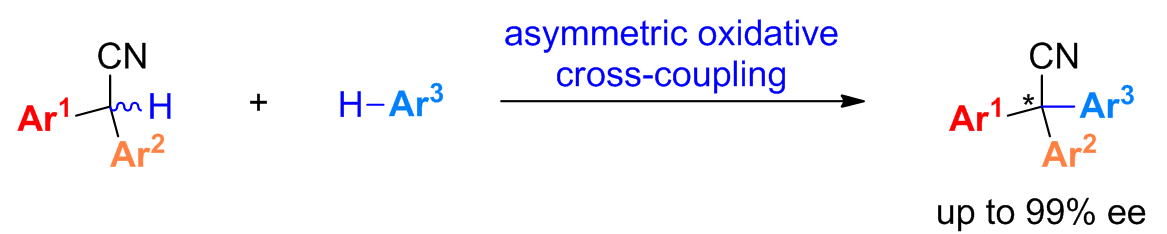
47. Synthesis of Chiral Triarylmethanes Bearing All-Carbon Quaternary Stereocenters: Catalytic Asymmetric Oxidative Cross-Coupling of 2,2-Diarylacetonitriles and (Hetero)arenes. Wang, Z.; Zhu, Y.; Pan, X.; Wang, G.; Liu, L.* Angew. Chem. Int. Ed. 2020, 59, 3053. link
46. Cross-dehydrogenative coupling of 3,6-dihydro-2H-pyrans with 1,3-dicarbonyls and aryl moieties. Feng, G.; Sun, C.; Xin, X.; Wan, R.*; Liu, L.* Tetrahedron Lett. 2019, 60, 1547. link
45. Oxidative C–H Alkylation of Naphthoquinones with Simple Alkenes. Cao, L.; Long, H.; Guan, H.; Bi, Y.; Bi, G.; Huang, H.* Liu, L.* Tetrahedron Lett. 2019, 60, 1268. link
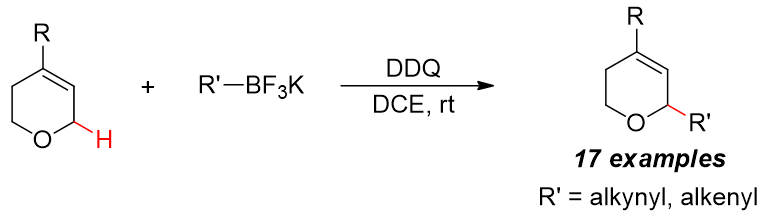
44. Oxidative C–H Alkynylation of 3,6-Dihydro-2H-pyrans. Zhao, R.; Feng, G.; Xin, X.; Guan, H.; Hua, J.; Wan, R.*; Li, W.*; Liu, L.* Chin. Chem. Lett. 2019, 30, 1432. link
43. Cross-Dehydrogenative Coupling of Secondary Benzylic Ethers with Indoles and Pyrroles. Cao, M.; Mao, Y.; Huang, J.; Ma, Y.*; Liu, L.* Tetrahedron Lett. 2019, 60, 1075. link
42. Direct Oxidative C(sp3)–H Cyanation of Secondary Benzylic Ethers. Wang, Z.; Mao, Y.; Guan, H.; Cao, M.; Hua, J.; Feng, L.*; Liu, L.* Chin. Chem. Lett. 2019, 30, 1241. link
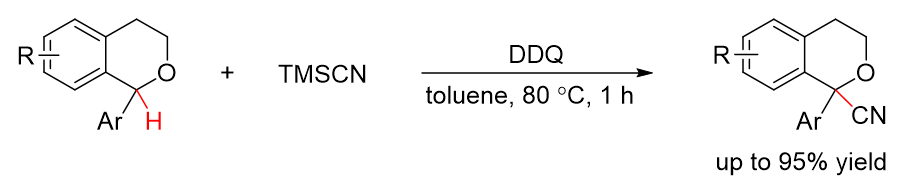
41. Bimolecular Oxidative C-H Alkynylation of α-Substituted Isochromans. Mao, Y.; Cao, M.; Pan, X.; Huang, J.; Li, J.; Xu, L.; Liu, L.* Org. Chem. Front. 2019, 6, 2028.link
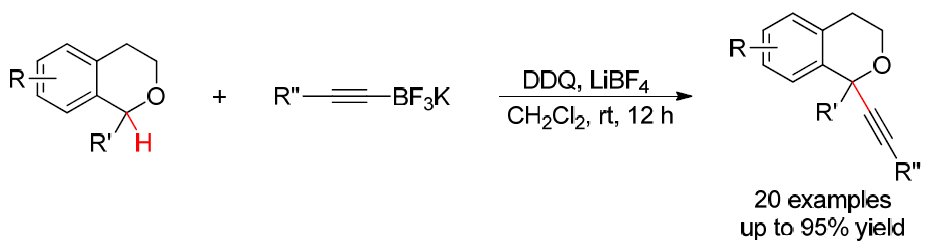
40. Iron-Catalyzed Aerobic Dehydrogenative Kinetic Resolution of Cyclic Secondary Amines. Lu, R.; Cao, L.; Guan, H.; Liu, L.* J. Am. Chem. Soc. 2019, 141, 6318. link
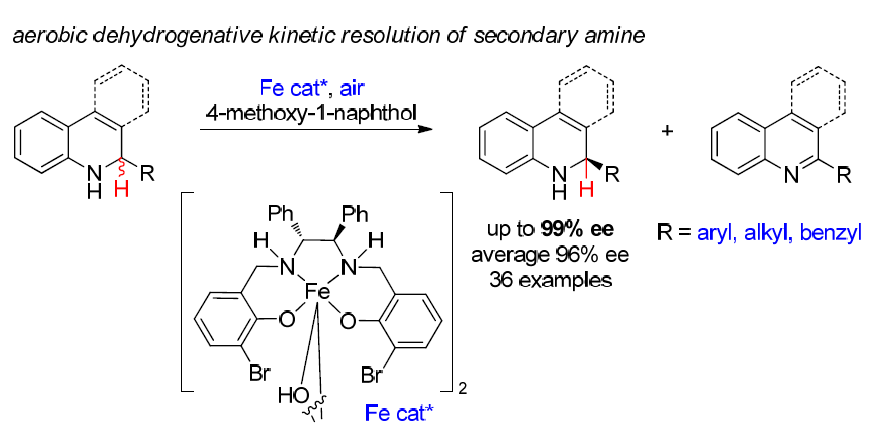
39. Catalytic Enantioselective Cross-Dehydrogenative Coupling of 3,6-Dihydro-2H-pyrans with Aldehydes. Xin, X.; Pan, X.; Meng, Z.; Liu, X.; Liu, L.* Org. Chem. Front. 2019, 6, 1448. link
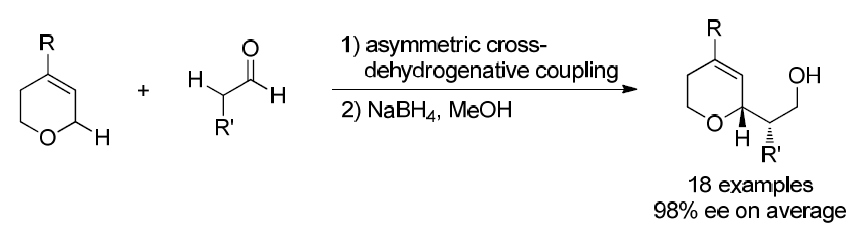
38. Catalytic Enantioselective Oxidative Coupling of Saturated Ethers with Carboxylic Acid Derivatives. Wang, G.; Xin, X.; Wang, Z.; Lu, G.; Ma, Y.; Liu, L.* Nature Commun. 10.1038/s41467-019-08473-x. link
(Featured in Nature Communications Editors‘ Highlights Webpage) link
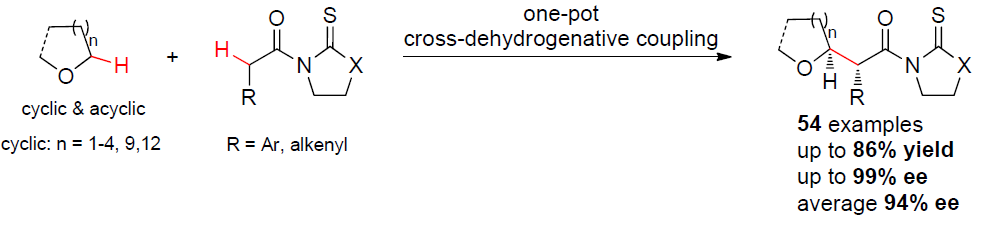
37. Three-Component Oxyarylation of Alkenes Enables Access to C3‑Substituted Dihydrobenzofurans. Feng, G.; Sun, S.; Liu, G.; Long, H.; Liu, L.* Org. Lett. 2018. 20, 7522. link (Top 20 most downloaded article for Nov and Dec 2018)
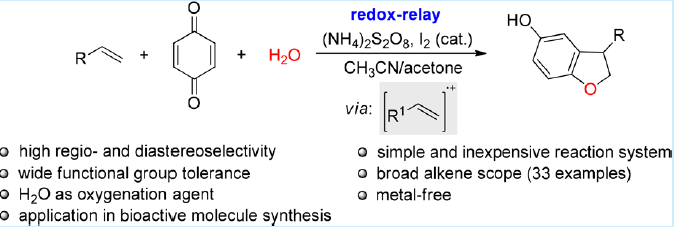
36. Metal‐free three‐component oxyalkynylation of alkenes. Li, Y.; Lu, R.; Sun, S.; Liu, L.* Org. Lett. 2018. 20, 6836. link Cited in Org. Chem. Highlights link
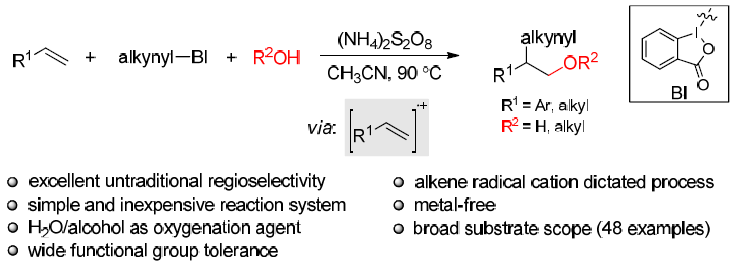
35. Pan, X.; Liu, X.; Sun, S.; Meng, Z.; Liu, L.* Catalytic Asymmetric Cross-Dehydrogenative Coupling of 2H-Chromenes and Aldehydes, Chin. J. Chem. 2018, 36, 1187. (Dedicated to Professor Xiyan Lu on the occasion of his 90th birthday) link
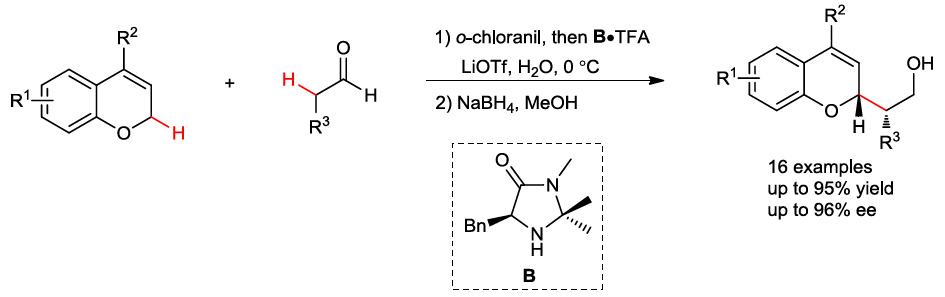
34. Iron(II)-catalyzed site-selective functionalization of unactivated C-H bonds guided by alkoxyl radical. Guan, H.; Sun, S.; Mao, Y.; Chen, L.; Lu, R.; Huang, J.; Liu, L.* Angew. Chem. Int. Ed. 2018, 57, 11413.link
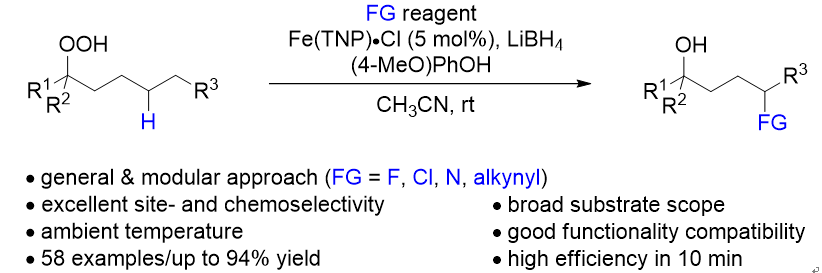
33. Copper-catalyzed oxidative cross-dehydrogenative coupling of 2H-chromenes and terminal alkynes. Yang, F.; Li, Y.; Floreancig, P. E.*; Li, X.*; Liu, L.* Org. Biomol. Chem. 2018, 16, 5144. link
32. 饱和开链醚的氧化碳氢炔基化研究. Guan, H.; Chen, L.; Liu, L.* Acta Chimica Sinica 2018, 76, 440. (invited contribution) link
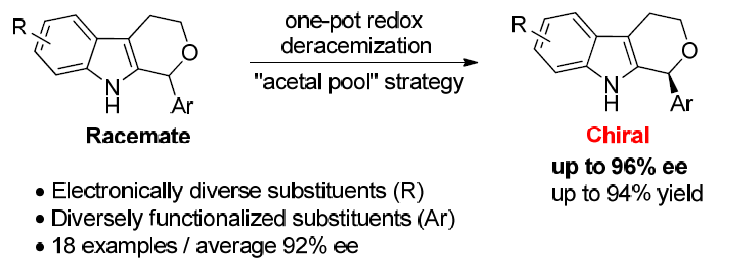
31. Redox Deracemization of 1,3,4,9‐Tetrahydropyrano[3,4‐b]indoles. Lu, R.; Li, Y.; Zhao, J.; Li, J.; Wang, S.; Liu, L.* Chem. Commun. 2018, 54, 4445. link
30. Direct Oxidative C-H Alkynylation of N-Carbamoyl Tetrahydroisoquinolines and Dihydroisoquinolines. Chen, L.; Sun, C.; Feng, G.; Cao, M.; Zhao, S.; Yan, J.; Wan, R.*; Liu, L.* Org. Biomol. Chem. 2018, 16, 2792. link
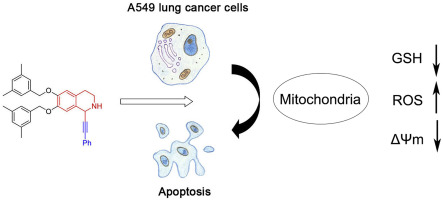
29. A Novel Tetrahydroisoquinoline (THIQ) Analogue Induces Mitochondria-dependent Apoptosis. Sun, X.; Liu, M.; Gao, L.; Mao, Y.; Zhao, D.; Zhang, J.; Liu, L.* Eur. J. Med. Chem. 2018, 150, 719. link.
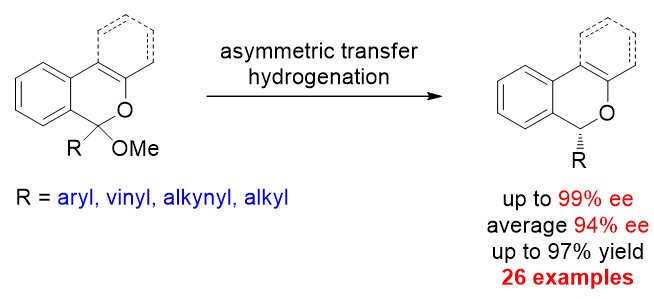
28. Efficient Access to Chiral Benzo[c]chromenes via Asymmetric Transfer Hydrogenation of Ketals. Li, Y.; Wan, M.; Sun, S.; Fu, Z.; Huang, H.; Liu, L.* Org. Chem. Front. 2018, 5, 1280. link.
27. Oxidative C-H functionalization of N-carbamoyl 1,2-Dihydroquinolines. Liu, Z.; Chen, L.; Li, J.; Liu, K.; Zhao, J.; Xu, M.; Feng, L.; Wan, R.; Li, W.; Liu, L.* Org. Biomol. Chem. 2017, 15, 7600. link
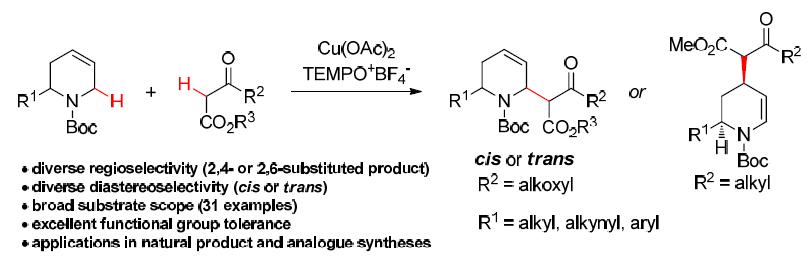
26. Regio‐ and Diastereoselective Cross‐Dehydrogenative Coupling of Tetrahydropyridines with 1,3‐Dicarbonyl Compounds, Long, H.; Wang, G.; Lu, R.; Xu, M.; Zhang, K.; Qi, S.; He, Y.; Bu, Y.; Liu, L.* Org. Lett. 2017, 19, 2146. link
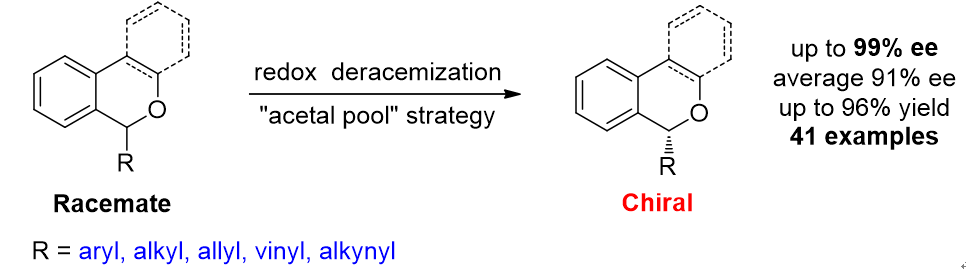
25. Organocatalytic Redox Deracemization of Cyclic Benzylic Ethers Enabled by An "Acetal Pool" Strategy, Wan, M.; Sun, S.; Li, Y.; Liu, L.* Angew. Chem. Int. Ed. 2017, 56, 5116. link
(featured inSynfacts 2017, 655)
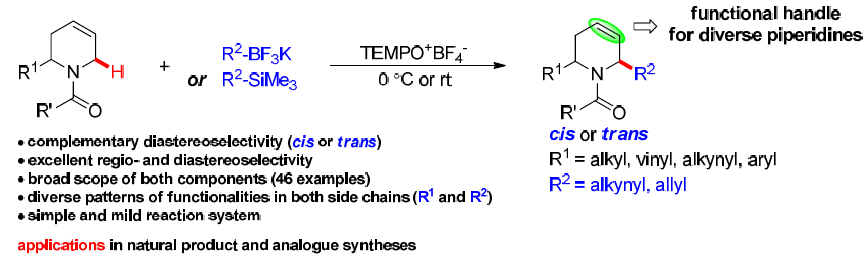
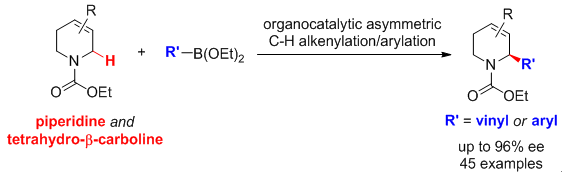
24. Diastereoselectively Complementary C-H Functionalization Enables Access to Structurally and Stereochemically Diverse 2,6-Substituted Piperidines. Wang, G.; Mao, Y.; Liu, L.* Org. Lett. 2016, 18, 6476. link
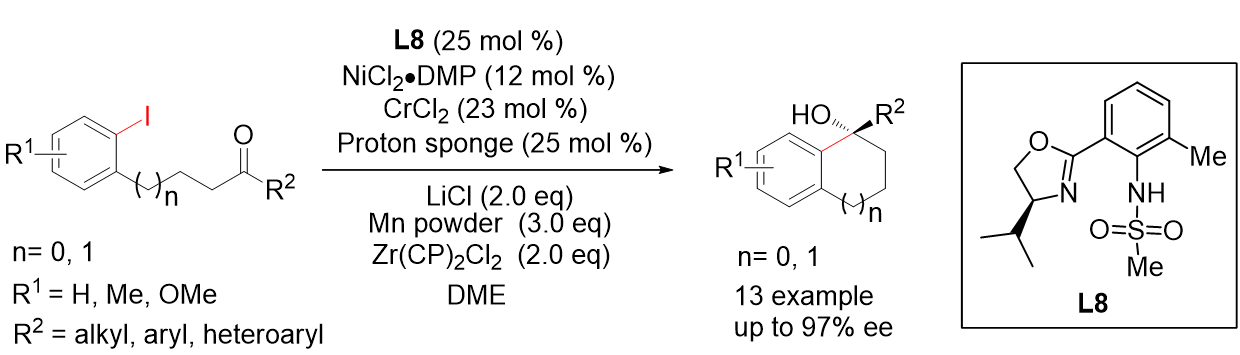
23. Chromium (II) Catalyzed Enantioselective Arylation of Ketones. Wang, G.; Sun, S.; Mao, Y.; Xie, Z.; Liu, L.* Beilstein. J. Org. Chem. 2016, 12, 2771. (Invited by Professor Tehshik P Yoon for Thematic Series "Strategies in Asymmetric Synthesis") link
22. A practical oxidative C-H functionalization of N-carbamoyl tetrahydro-beta-carbolines with diverse potassium trifluoroborates. Sun, Y.; Wang, G.; Chen, J.; Liu, C.; Cai, M.; Zhu, R.; Huang, H.; Li, W.; Liu, L.* Org. Biomol. Chem. 2016, 14, 9431. link
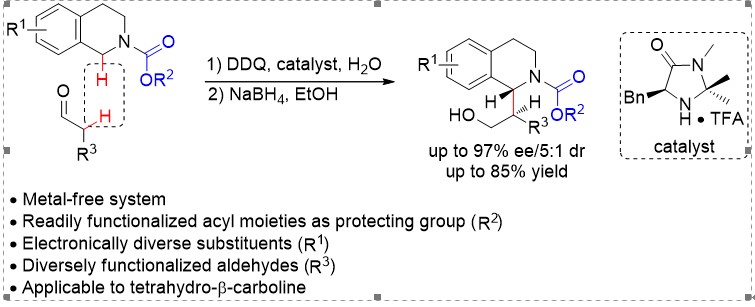
21. Organocatalytic Enantioselective Cross-Dehydrogenative Coupling of N-Carbamoyl Cyclic Amines with
Aldehydes. Xie, Z.; Zan, X.; Sun, S.; Pan, X.; Liu, L.* Org. Lett. 2016, 18, 3944. Link
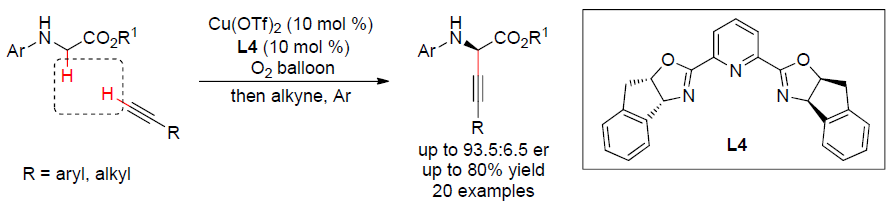
20. Copper-Catalyzed Aerobic Enantioselective Cross-Dehydrogenative Coupling of N-Aryl Glycine Esters with Terminal Alkynes. Xie, Z.; Liu, X.; Liu, L.* Org. Lett. 2016, 18, 2982. link (featured inSynfacts 2016, 948)
19. Catalytic Enantioselective Alkynylation of Tetrahydroisoquinoline-Based N-Acyl Hemiaminals. Sun, S.; Liu, L.* Synthesis, 2016, 48, 2627. (Invited by Professor Erick Carreira for special topic "Asymmetric Synthesis") link
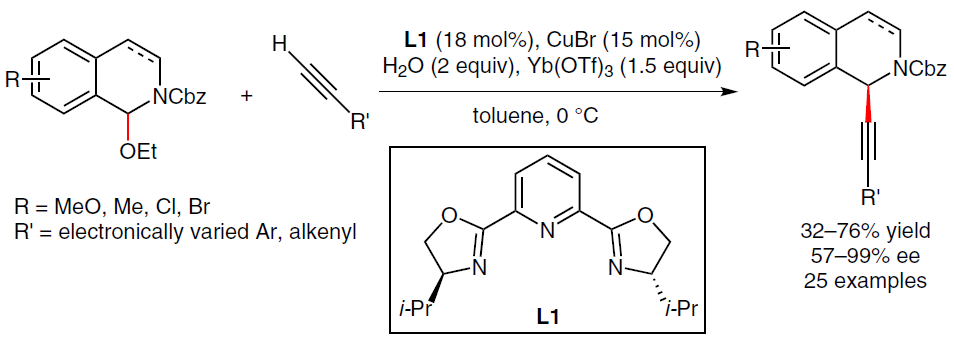
18. Copper(II) Triflate-Catalyzed Aerobic Oxidative C–H Functionalization of Glycine Derivatives with Olefins and Organoboranes. Xie, Z.; Jia, J.; Liu, X.; Liu, L.* Adv. Synth. Catal. 2016, 358, 919. link
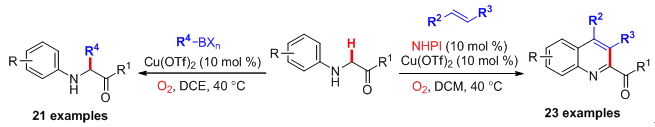
17. An Economical Synthesis of Substituted Quinoline-2-Carboxylates through Potassium Persulfate-Mediated Cross-Dehydrogenative Coupling of N-Aryl Glycine Derivatives with Olefins. Liu, G.; Qian, J.; Hua, J.; Cai, F.; Li, X.; Liu. L.* Org. Biomol. Chem. 2016, 14, 1147. link
16. TBHP/TFA Mediated Oxidative Cross-Dehydrogenative Coupling of N-Heterocycles with Aldehydes. Chen, J.; Wan, M.; Hua, J.; Sun, Y.; Lv, Z.; Li, W.; Liu, L.* Org. Biomol. Chem .2015, 13, 11561. link (featured in Synfacts 2016, 16)
15. C1-Benzyl and Benzoyl Isoquinoline Synthesis through Direct Oxidative Cross-Dehydrogenative Coupling with Methyl Arenes. Wan, M.; Lou,H.; Liu, L.* Chem. Commun., 2015, 51, 13953. link (featured in Synfacts 2015, 13953)
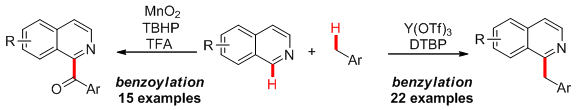
14. Copper(II)/Amine Synergistically Catalyzed Enantioselective Alkylation of Cyclic N-Acyl Hemiaminals with Aldehydes. Sun, S.; Mao,Y.; Lou, H.; Liu, L.* Chem. Common., 2015, 51, 10691. link
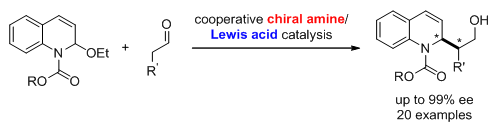
13. Organocatalytic Asymmetric C-H Vinylation and Arylation of N-Acyl Tetrahydroisoquinolines. Liu, X.; Sun,S.; Meng, Z.; Lou, H.; Liu, L.* Org. Lett. 2015, 17, 2396. link (Top 20 most downloaded article for May 2015)

12. Indium-Catalyzed Oxidative Cross-Dehydrogenative Coupling of Chromenes with 1,3-Dicarbonyls and Aryl Rings. Li, F.; Meng, Z.; Hua, J.; Li, W.; Lou, H.; Liu, L.* Org. Biomol. Chem. 2015, 13, 5710. link
11. Highly Enantioselective Catalytic Cross-Dehydrogenative Coupling of N-Carbamoyl Tetrahydroisoquinolines and Terminal Alkynes. Sun, S.; Li, C.; Floreancig, P. E.; Lou, H.; Liu, L.* Org. Lett. 2015, 17, 1684. link (featured in Synfacts 2015, 633; Top 1 most downloaded article for May 2015)
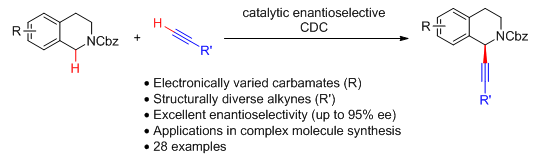
10. Organocatalytic Enantioselective Oxidative C-H Alkenylation and Arylation of N-Carbamoyl Tetrahydropyridines and Tetrahydro-β-Carbolines. Liu, X.; Meng, Z.; Li, C.; Lou, H.; Liu, L.* Angew. Chem. Int. Ed. 2015, 54, 6012. link (featured in Synfacts 2015, 661; cited in Org. Chem. highlights, link)
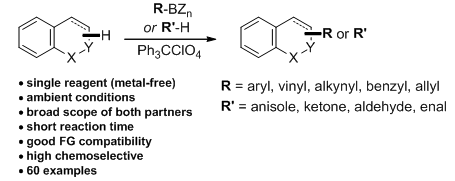
9. Structurally Diverse α-Substituted Benzopyran Synthesis through A Practical Metal-Free C(sp3)-H Functionalization. Chen, W.;Xie, Z.; Zheng, H.; Lou, H.*; Liu, L.* Org. Lett. 2014, 16, 5988. link
8. Seven- and Eight-Membered Heterocyclic Biaryl Synthesis through A Metal-Free Oxidative Coupling Reaction. Sun, S.; Yang, J.; Li, F.; Lv, Z.; Li, W.; Lou, H.*; Liu, L.* Tetrahedron Lett .2014, 55, 6899. link
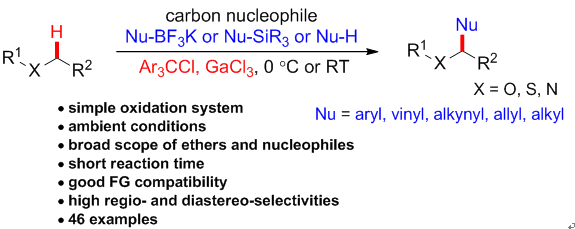
7. Practical and Highly Selective C-H Functionalizationof Structurally Diverse Ethers. Wan, M.; Meng,Z.; Lou, H.; Liu, L.* Angew. Chem. Int. Ed. 2014, 53, 13845. link (selected as Hot Paper by Angew)
6. An Economic and Environmentally Friendly Oxidative Biaryl Coupling Promoted
by Activated MnO2. Yang, J.; Sun, S.; Zeng, Z.; Zheng, H.; Lou, H.; Liu,
L.* Org. Biomol. Chem. 2014, 12, 7774. link
5. Copper(II) Catalyzed Cross-Dehydrogenative Coupling of Cyclic Benzylic Ethers With Simple Carbonyl Compounds by Na2S2O8. Pan, X.; Hu, Q.; Chen, W.; Liu, X.; Sun, B.; Huang, Z.; Zeng, Z.; Wang, L.; Zhao, D.; Ji, M.; Liu, L.*; Lou, H*. Tetrahedron 2014, 70, 3447. link
4. A Metal-Free Cross-Dehydrogenative Coupling of N-Carbamoyl Tetrahydroispquinolineby Sodium Persulfate. Chen, W.; Zheng, H.; Pan, X.; Xie, Z.; Zan, X.; Sun, B.; Liu, L.*;Lou, H.* Tetrahedron Lett. 2014, 55, 2879. link
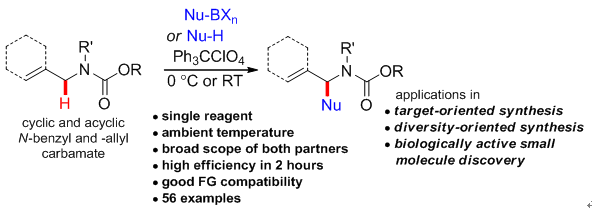
3. Practical Metal-Free C(sp3)-H Functionalization: Construction of Structurally Diverse a-Substituted N-Benzyl and N-Allyl Carbamates. Xie, Z.; Liu, L.*; Chen, W.; Zheng, H.; Xu, Q.; Yuan, H.; Lou, H.* Angew. Chem. Int. Ed .2014, 53, 3904. link (Highlight by Chinese J Org Chem)
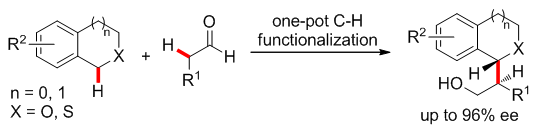
2. Catalytic Enantioselective Oxidative Cross-Coupling of Benzylic Ethers with Aldehydes. Meng, Z.; Sun, S.; Yuan, H.; Lou, H.*; Liu, L.* Angew. Chem. Int. Ed .2014, 53, 543. link (selected as Hot Paper by Angew; highlighted by Synfacts)
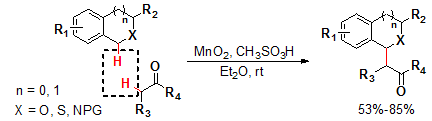
1. Manganese Dioxide-Methanesulfonic Acid Promoted Direct Dehydrogenative Alkylation
of sp3 C–H Bonds Adjacent to A Heteroatom. Liu, X.; Sun, B.; Xie, Z.; Qin, X.; Liu, L.*;
Lou, H.X.* J. Org. Chem. 2013, 78, 3104. link
Selected Graduate and Postdoctoral Work
13. Synthesis of alcohols from m-Fluorophenylsulfones and Dialkylboranes: Application to theC14-C35 Building Block of E7389. Liu, L.; Henderson, J. A.; Yamamoto, A.; Brémond, P.; Kishi, Y. Org. Lett. 2012, 14, 2262.
12. Stereoselective Synthesis of Tertiary Ethers through Geometrical Control of Highly Substituted Oxocarbenium Ions. Liu, L.; Floreancig, P. E. Angew. Chem. Int. Ed. 2010, 49, 5894. (Featured in SynFacts 2010, 1152 and Synstory 2010, A101)
11. Structurally and Stereochemically Diverse Tetrahydropyran Synthesis through Oxidative Carbon–Hydrogen Bond Activation. Liu, L.; Floreancig, P. E. Angew. Chem. Int. Ed. 2010, 49, 3069. (Selected as “Hot Paper” in Angew)
10. Stereoselective Heterocycles Synthesis through OxidativeCarbon–Hydrogen Bond Activation. Liu, L.; Floreancig, P. E. Curr. Opin. Drug. Discov. Devel. 2010, 13, 733.
9. DDQ-Catalyzed Reactions Employing MnO2 as A Stoichiometric Oxidant. Liu, L.; Floreancig, P. E. Org. Lett. 2010, 12, 4686.
8. Cyclization Reactions through DDQ-Mediated Vinyl Oxazolidinone Oxidation. Liu, L.; Floreancig, P. E. Org. Lett. 2009, 11, 3152. (Featured in SynFacts 2009, 998)
7. Diastereoselective Tetrahydropyrone Synthesis through Transition-Metal-Free Oxidative Carbon–Hydrogen Bond Activation. Tu, W.; Liu, L.; Floreancig, P. E. Angew. Chem. Int. Ed. 2008, 47, 4184.
6. Highly Enantioselective Addition of Phenylacetylene to Ketones Catalyzed by Bis(hydroxycamphorsulfonamide)–Copper(II) Complex. Liu, L.; Wang, R.; Kang, Y.-F.; Cai, H.-Q.; Chen, C. Synlett. 2006, 8, 1245.
5. Low Ligand Loading, Highly Enantioselective Addition of Phenylacetylene to Aromatic Ketones Catalyzed by Schiff-Base Amino Alcohols. Chen, C.; Hong, L.; Xu, Z.-Q.; Liu, L.; Wang, R. Org. Lett. 2006, 8, 2277.
4. Enantioselective Alkynylation of Aromatic Ketones Catalyzed by New Chiral Oxazolidine Ligands. Kang, Y.-F.;Liu, L.; Wang, R.; Zhou, Y.-F.; Yan, W.-J. Adv. Synth. Catal. 2005, 347, 243.
3. Highly Enantioselective Phenylacetylene Addition to Aromatic KetonesCatalyzed by Cinchona Alkaloid-Aluminum Complexes. Liu, L.; Wang, R.; Kang, Y.-F.; Chen, C.; Xu, Z.-Q.; Zhou, Y.-F.; Ni, M.; Cai, H.-Q.; Gong, M.-Z. J. Org. Chem. 2005, 70, 1084.
2. Highly Enantioselective Phenylacetylene Additions to Ketones Catalyzed by (S)-BINOL-Ti Complex. Zhou, Y.-F.; Wang, R.; Xu, Z.-Q.; Yan, W.-J.; Liu, L.; Kang, Y.-F.; Han, Z.-J. Org. Lett. 2004, 6, 4147.
1. Enantioselective Alkynylation of Aromatic Ketones Promoted by (S)-Phenylalanine-derived β-Amino Alcohol. Liu, L.; Kang, Y.-F.; Wang, R.; Zhou, Y.-F.; Chen, C.; Ni, M.; Gong, M.-Z. Tetrahedron: Asymmetry 2004, 15, 3757.